Product Pipeline
Group B Streptococcal (GBS) Vaccine for prevention of life-threatening infections in newborns.
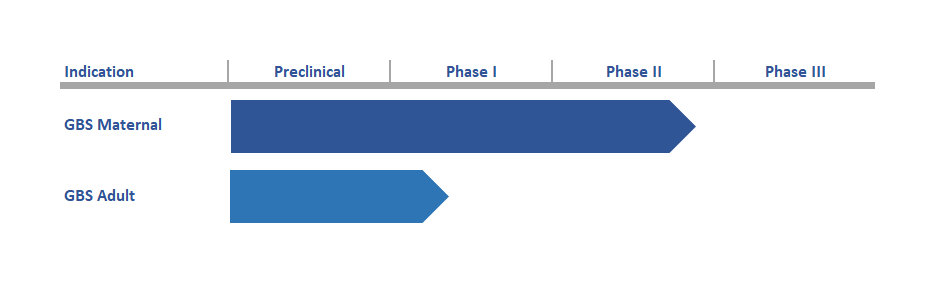
MinervaX is engaged in clinical development of a Group B Streptococcal vaccine targeting pregnant women for the prevention of life-threatening infections in newborns. So far the vaccine has been dosed to 240 healthy adult women. The vaccine has proven safe, highly immunogenic and giving rise to functionally active antibodies.
MinervaX is developing a novel innovative vaccine candidate against Group B Streptococcus.
MinervaX is developing a novel innovative, adjuvanted protein-only vaccine based on bacterial antigens derived from the family of alpha-like surface proteins of Group B Streptococcus. The antigens are based on the N-terminal domains of the most prevalent alpha-like protein serotypes (AlpCN, RibN, Alp1N and Alp2/3N), covering close to 100% of clinical GBS isolates. The vaccine consists of two fusion proteins consisting of either AlpCN and RibN (GBS-NN) or Alp1N and Alp2/3N (GBS-NN2), formulated with AlOH adjuvant (1). MinervaX believes its vaccine is likely to have superior characteristics compared with other GBS vaccine candidates in development, which are based on traditional capsular polysaccharide (CPS) conjugate technology.
The GBS-NN vaccine component has demonstrated efficacy in animal models of lethal GBS infections: passive immunization models, active immunization and neonatal protection models (2–5). Naturally occurring antibodies against the vaccine antigens are present in most individuals, originating from colonization with the bacteria already from an early age. The naturally occurring antibodies are IgG1 and accumulate in the fetus due to active placental transfer from mother to child, reaching some 120% in the infant compared to the mother. Naturally occurring antibodies against the vaccine antigens have been found to correlate with protection against invasive GBS disease in infants (6), and preliminary correlates of protection have been developed. The correlates indicates that antibodies against the vaccine antigens are protective and the protective thresholds provides guidance in terms of the levels of vaccine induced antibodies needed to confer protection in vaccinated individuals.
An initial Phase I trial of the GBS-NN component alone in 240 healthy adult volunteers demonstrated that the vaccine had a safety profile at par with other AlOH adjuvanted protein-based vaccine, with no safety concerns being raised. The vaccine induced high levels of long-lasting functionally active antibodies, capable of both blocking the invasion of epithelial cells with GBS (a key step for establishment of invasive GBS infection) and killing GBS once entering the body via opsono-phagocytosis. A subsequent Phase I trial in 60 healthy adult women demonstrated equal safety of the GBS-NN & GBS-NN2 combination. This trial also documented high levels of antibodies against all 4 N-terminal domains, and that 100% of vaccinated subjects reached the predicted correlates of protection derived from case-control studies of naturally occurring antibodies in infants contracting invasive GBS disease and relevant controls. High opsonophagocytic titres were also obtained in all vaccinated individuals against GBS isolates expressing all vaccine antigens, confirming the close to 100% coverage against clinical GBS isolates.
The vaccine is currently in Phase II development. A clinical study in 200 pregnant women living with or without HIV in South Africa and Uganda has completed enrolment, and 140+ babies have been delivered to date. The study is supported by an EDCTP grant from the European Union. Furthermore, a clinical study in 270 healthy pregnant women has been initiated in Denmark and United Kingdom. The study is conducted under an IND.
In addition to pregnant women, the vaccine is also being explored for use in older adults, where GBS cause significant disease such as sepsis, pneumonia, cellulitis and soft tissue infections.
- Lindahl, G., M. Stålhammar-Carlemalm, and T. Areschoug. 2005. Surface proteins of Streptococcus agalactiae and related proteins in other bacterial pathogens. Clin Microbiol Rev 18:102-127.
- Larsson, C., M. Stålhammar-Carlemalm, and G. Lindahl. 1996. Experimental vaccination against group B streptococcus, an encapsulated bacterium, with highly purified preparations of cell surface proteins Rib and alpha. Infect Immun 64:3518-3523.
- Larsson, C., M. Stålhammar-Carlemalm, and G. Lindahl. 1999. Protection against experimental infection with group B streptococcus by immunization with a bivalent protein vaccine. Vaccine 17:454-458.
- Stålhammar-Carlemalm, M., L. Stenberg, and G. Lindahl. 1993. Protein Rib: a novel group B streptococcal cell surface protein that confers protective immunity and is expressed by most strains causing invasive infections. J Exp Med 177:1593-1603.
- Stålhammar-Carlemalm, M., J. Waldemarsson, E. Johnsson, T. Areschoug, and G. Lindahl. 2007. Nonimmunodominant regions are effective as building blocks in a streptococcal fusion protein vaccine. Cell Host Microbe 2:427-434.
- Larsson, C., M. Lindroth, P. Nordin, M. Stålhammar-Carlemalm, G. Lindahl, and I. Krantz. 2006. Association between low concentrations of antibodies to protein alpha and Rib and invasive neonatal group B streptococcal infection. Arch Dis Child Fetal Neonatal Ed 91:F403-408.
